Journal Description
Scientia Pharmaceutica
Scientia Pharmaceutica
is an international, peer-reviewed, open access journal related to the pharmaceutical sciences. The journal is owned by the Austrian Pharmaceutical Society (Österreichische Pharmazeutische Gesellschaft, ÖPhG) and is published quarterly online by MDPI and in print by the Austrian Pharmacists' Publishing House (Österreichischer Apothekerverlag).
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within Scopus, ESCI (Web of Science), Embase, CAPlus / SciFinder, and other databases.
- Journal Rank: CiteScore - Q2 (Pharmaceutical Science)
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 22.7 days after submission; acceptance to publication is undertaken in 6.9 days (median values for papers published in this journal in the second half of 2023).
- Recognition of Reviewers: reviewers who provide timely, thorough peer-review reports receive vouchers entitling them to a discount on the APC of their next publication in any MDPI journal, in appreciation of the work done.
Impact Factor:
2.5 (2022);
5-Year Impact Factor:
3.5 (2022)
Latest Articles
Wide Use of Hyaluronic Acid in the Process of Wound Healing—A Rapid Review
Sci. Pharm. 2024, 92(2), 23; https://doi.org/10.3390/scipharm92020023 - 25 Apr 2024
Abstract
Hyaluronic acid (HA), as one of the main components of the extracellular matrix (ECM), plays an important role in the process of wound-healing and tissue-repair processes due to its unique properties and different physiological functions. HA has an ability to maintain a moist
[...] Read more.
Hyaluronic acid (HA), as one of the main components of the extracellular matrix (ECM), plays an important role in the process of wound-healing and tissue-repair processes due to its unique properties and different physiological functions. HA has an ability to maintain a moist environment that promotes healing, the stimulation of growth factors and cellular constituents, and the migration of various cells essential for healing. This paper offers a review of HA use in the process of wound healing, with emphasis on hard-to-heal wounds, and examines its various applications in ophthalmology and otorhinolaryngology. It proves HA to be a versatile agent which finds its use in various fields of medicine for its antioxidant, anti-inflammatory, antibacterial properties and accelerated wound healing.
Full article
(This article belongs to the Special Issue Feature Papers in Scientia Pharmaceutica)
Open AccessArticle
Stability-Indicating UPLC-PDA-QDa Methodology for Carvedilol and Felodipine in Fixed-Dose Combinations Using AQbD Principles
by
Jesús Alberto Afonso Urich, Viktoria Marko, Katharina Boehm, Raymar Andreina Lara Garcia, Anna Fedorko, Sharareh Salar-Behzadi and Dalibor Jeremic
Sci. Pharm. 2024, 92(2), 22; https://doi.org/10.3390/scipharm92020022 - 25 Apr 2024
Abstract
The development of analytical procedures, in line with the recent regulatory requirements ICH Q2 (R2) and ICH Q14, is progressing, and it must be able to manage the entire life cycle of the methodology. This is also applicable to and especially challenging for
[...] Read more.
The development of analytical procedures, in line with the recent regulatory requirements ICH Q2 (R2) and ICH Q14, is progressing, and it must be able to manage the entire life cycle of the methodology. This is also applicable to and especially challenging for combinations of drug substances and dosage form. A reliable and efficient, stability-indicating, MS-compatible, reverse-phase ultra-performance liquid chromatographic (UPLC®) method was developed for the determination of carvedilol and felodipine in a combination oral dosage form. The development of the method, performed using analytical quality by design (AQbD) principles, was in line with the future regulatory requirements. Furthermore, the fixed-dose combination dosage forms are a clear solution to the polypharmacy phenomenon in the elderly population. The main factors evaluated were the mobile phase buffer, organic modifier, column, flow, and column temperature. The optimum conditions were achieved with a Waters Acquity HSS T3 (100 × 2.1 mm i.d., 1.8 µm) column at 38 °C, using ammonium acetate buffer (5 mM, pH 4.5) (Solution A) and MeOH (Solution B) as mobile phases in gradient elution (t = 0 min, 10% B; t = 1.5 min, 10% B; t = 12.0 min, 90% B; t = 13.0 min, 10% B; t = 15.5 min, 10% B) at a flow rate of 0.2 mL/min and UV Detection of 240 and 362 nm for carvedilol (CAV) and felodipine (FLP), respectively. The linearity was demonstrated over concentration ranges of 30–650 µg/mL (R2 = 0.9984) (CAV) and 32–260 µg/mL (R2 = 0.9996) (FLP). Forced degradation studies were performed by subjecting the samples to hydrolytic (acid and base), oxidative, and thermal stress conditions. Standard solution stability was also performed. The proposed validated method was successfully used for the quantitative analysis of bulk, stability, and fixed-dose combination dosage form samples of the desired drug product. Using the AQbD principles, it is possible to generate methodologies with improved knowledge, leading to high-quality data, lower operation costs, and minimum regulatory risk. Furthermore, this work paves the way for providing a platform of robust analytical methods for the simultaneous quantification of innovative on-demand new dose combinations.
Full article
(This article belongs to the Special Issue Feature Papers in Scientia Pharmaceutica)
►▼
Show Figures
Graphical abstract
Open AccessArticle
Design, Synthesis and Antimicrobial Potential of Conjugated Metallopeptides Targeting DNA
by
Maria Camila Moreno-Ramirez, Adriana Stefania Arias-Bravo, Alberto Aragón-Muriel, César Alonso Godoy, Yamil Liscano, Jose Oñate Garzón and Dorian Polo-Cerón
Sci. Pharm. 2024, 92(2), 21; https://doi.org/10.3390/scipharm92020021 - 17 Apr 2024
Abstract
Antimicrobial resistance threatens the effective prevention and treatment of an increasingly broad spectrum of infections caused by pathogenic microorganisms. This pressing challenge has intensified the search for alternative antibiotics with new pharmacological properties. Due to the chemical synergy between the biological activity of
[...] Read more.
Antimicrobial resistance threatens the effective prevention and treatment of an increasingly broad spectrum of infections caused by pathogenic microorganisms. This pressing challenge has intensified the search for alternative antibiotics with new pharmacological properties. Due to the chemical synergy between the biological activity of antimicrobial peptides (AMPs) and the different modes of action, catalytic properties, and redox chemistry of metal complexes, metallopeptides have emerged in recent years as an alternative to conventional antibiotics. In the present investigation, peptide ligands conjugated with 5-carboxy-1,10-phenanthroline (Phen) were prepared by solid-phase peptide synthesis (SPPS), and the corresponding copper(II) metallopeptides, Cu-PhenKG and Cu-PhenRG (where K = lysine, R = arginine, and G = glycine), were synthesized and characterized. The antimicrobial activities of these compounds toward Gram-positive and Gram-negative bacteria, evaluated by the broth microdilution technique, indicate that the metal center in the metallopeptides increases the antimicrobial activity of the complexes against the conjugated peptide ligands. Minimum inhibitory concentration (MIC) values of 0.5 μg/mL for S. aureus with the Cu-PhenKG complex and 0.63 μg/mL for S. typhimurium with the Cu-PhenRG complex were obtained. The MIC values found for the conjugated peptides in all microorganisms tested were greater than 1.5 μg/mL. The interactions of the conjugated peptides and their metallopeptides with plasmid DNA were evaluated by agarose gel electrophoresis. Alterations on the replication machinery were also studied by polymerase chain reaction (PCR). The results indicate that the complexes interact efficiently with pBR322 DNA from E. coli, delaying the band shift. Furthermore, the resulting DNA–metallopeptide complex is not a useful template DNA because it inhibits PCR, since no PCR product was detected. Finally, molecular dynamics and molecular docking simulations were performed to better understand the interactions of the obtained compounds with DNA. The Cu-PhenRG complex shows a significantly higher number of polar interactions with DNA, suggesting a higher binding affinity with the biopolymer.
Full article
(This article belongs to the Special Issue Feature Papers in Scientia Pharmaceutica)
►▼
Show Figures
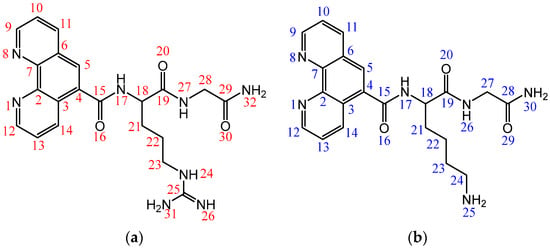
Figure 1
Open AccessCommunication
Nanosized Particles of Synthetic Silicon Dioxide Delay the Regeneration of Gastric Ulcers Created by N-Methyl-N′-Nitro-N-Nitrosoguanidine and Induce Hyper-Trophic Gastritis-like Symptoms
by
Ayaka Iwasaki, Yuichi Kawai and Akira Onodera
Sci. Pharm. 2024, 92(2), 20; https://doi.org/10.3390/scipharm92020020 - 11 Apr 2024
Abstract
►▼
Show Figures
Synthetically produced silicon dioxide used as a food additive exhibits nanoparticle size and shape during the early stages of manufacturing. Even when processed into food products, these nanoparticles are detected. Although processing food ingredients into nanoparticles can improve absorption rates or enhance texture,
[...] Read more.
Synthetically produced silicon dioxide used as a food additive exhibits nanoparticle size and shape during the early stages of manufacturing. Even when processed into food products, these nanoparticles are detected. Although processing food ingredients into nanoparticles can improve absorption rates or enhance texture, there are concerns about the specific biological effects of nanoparticles. In this study, three types of silica particles, including nanosized particles, were repetitively administered to the stomach using a gastric tube or exposed to a single injection into the submucosal layer of the stomach. Macroscopic and microscopic examinations did not reveal acute toxicity. However, when silica particles were administered to the stomach during the healing and regeneration process of gastric ulcers (induced by injecting the alkylating agent of N-Methyl-N′-Nitro-N-Nitrosoguanidine into the submucosal layer), silica particles with a diameter of 70 nm (SiNPs-70) delayed regeneration more strongly than microsized silica particles with diameters of 300 nm or 1000 nm (SiMPs-300, -1000). Furthermore, fibrosis for tissue regeneration spread throughout the entire mucosa of the stomach, resulting in hypertrophic gastritis-like symptoms. The frequency of this symptom was over 50% with SiNPs-70, 20% with SiMPs-300, and 0% with SiMPs-1000. Although the silica particles used in this study differ from actual samples found in food, the impact of particle size, particularly the effects unique to nanosize, was identified as toxicity in the stomach healing process.
Full article

Graphical abstract
Open AccessArticle
β-Sitosterol Mediates Gastrointestinal Smooth Muscle Relaxation Induced by Coccoloba uvifera via Muscarinic Acetylcholine Receptor Subtype 3
by
Francisco J. Aguirre-Crespo, José L. Aragón-Gastélum, Eduardo J. Gutiérrez-Alcántara, Pedro Zamora-Crescencio, Diana L. Gómez-Galicia, Diego R. Alatriste-Kurzel, Guzman Alvarez and Emanuel Hernández-Núñez
Sci. Pharm. 2024, 92(2), 19; https://doi.org/10.3390/scipharm92020019 - 05 Apr 2024
Abstract
Coccoloba uvifera is a Mayan medicinal plant, and these leaves are used as antidiarrheal and diuretic agents. In the present work, we develop in-vitro, ex-vivo, in-vivo, and in-silico strategies to evaluate several aqueous extracts of C. uvifera leaves. In vitro tests showed that
[...] Read more.
Coccoloba uvifera is a Mayan medicinal plant, and these leaves are used as antidiarrheal and diuretic agents. In the present work, we develop in-vitro, ex-vivo, in-vivo, and in-silico strategies to evaluate several aqueous extracts of C. uvifera leaves. In vitro tests showed that decoction extract (CuDe) presented the best yield and chlorophyll, phenol, and flavonoid content; however, CuDe showed low antioxidant activity (DPPH model). All aqueous extracts exert spasmolytic and vasorelaxant activity in a concentration-dependent manner (ex vivo), and in vivo tests showed that CuDe exerts the best antiperistaltic and diuretic effects. The in-silico analysis suggests that C. uvifera triterpenes act as a ligand of GPCR, and β-sitosterol could act as an antagonist of muscarinic acetylcholine receptor subtype 3 (m3AChR). In the context of aqueous extracts of C. uvifera, β-sitosterol and their heterosides were identified by FTIR and 1H-NMR spectroscopy. The concerted binding of β-sitosterol and other triterpenes within the m3AChR binding site may be relevant for the induction of relaxant effects at the gastrointestinal smooth muscle level. In this context, C. uvifera is a high-value plant species that requires analytical and pharmacological studies to confirm traditional medicinal use.
Full article
(This article belongs to the Topic Natural Products and Drug Discovery)
►▼
Show Figures
Figure 1
Open AccessArticle
Synthesis, Biological Evaluation, Molecular Docking and ADME Studies of Novel Pyrrole-Based Schiff Bases as Dual Acting MAO/AChE Inhibitors
by
Emilio Mateev, Magdalena Kondeva-Burdina, Maya Georgieva, Alexandrina Mateeva, Iva Valkova, Virginia Tzankova and Alexander Zlatkov
Sci. Pharm. 2024, 92(2), 18; https://doi.org/10.3390/scipharm92020018 - 29 Mar 2024
Abstract
►▼
Show Figures
Considering the complex pathogenesis of Alzheimer’s disease (AD), the multitarget ligand strategy is expected to provide superior effects for the treatment of the neurological disease compared to the classic single target approach. Thus, a series of 13 novel (5e-q) pyrrole-based Schiff
[...] Read more.
Considering the complex pathogenesis of Alzheimer’s disease (AD), the multitarget ligand strategy is expected to provide superior effects for the treatment of the neurological disease compared to the classic single target approach. Thus, a series of 13 novel (5e-q) pyrrole-based Schiff bases were synthesized by conventional and microwave-assisted condensations, and the compounds were evaluated for MAO-A, MAO-B and AChE inhibitory activities. The chemical structures of the newly formed molecules were elucidated by a combination of spectral methods. The obtained results confirmed the theoretical data. The majority of the title Schiff bases demonstrated good potential towards AChE at 10 μM concentrations, with the most promising compound 5m (58%) exerting a comparative effect to that of the applied standard—Donepezil. 5j and 5o selectively inhibited MAO-B by 26% and 21% (at 1 μM concentration), respectively. The compound condensed with 5-nitro-2-furaldehyde (5j) achieved the best dual MAO-B and AChE inhibitory capacities. In addition to the in vitro analysis, docking simulations targeting the active sites of AChE (PDB ID: 4EY6) and MAO-B (PDB: 2V5Z) were employed to explore the possible interactions of the most prominent dual inhibitor (5j) with the enzymes. Furthermore, in silico ADME and PAMPA-blood–brain barrier (BBB) studies were conducted.
Full article
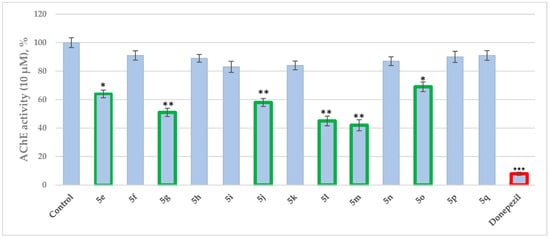
Figure 1
Open AccessArticle
Semaglutide as a Possible Calmodulin Binder: Ligand-Based Computational Analyses and Relevance to Its Associated Reward and Appetitive Behaviour Actions
by
Giuseppe Floresta, Davide Arillotta, Valeria Catalani, Gabriele Duccio Papanti Pelletier, John Martin Corkery, Amira Guirguis and Fabrizio Schifano
Sci. Pharm. 2024, 92(2), 17; https://doi.org/10.3390/scipharm92020017 - 22 Mar 2024
Abstract
Semaglutide, a glucagon-like peptide-1 (GLP-1) receptor agonist, has gained considerable attention as a therapeutic agent for type 2 diabetes mellitus and obesity. Despite its clinical success, the precise mechanisms underlying its pharmacological effects remain incompletely understood. In this study, we employed ligand-based drug
[...] Read more.
Semaglutide, a glucagon-like peptide-1 (GLP-1) receptor agonist, has gained considerable attention as a therapeutic agent for type 2 diabetes mellitus and obesity. Despite its clinical success, the precise mechanisms underlying its pharmacological effects remain incompletely understood. In this study, we employed ligand-based drug design strategies to investigate potential off-target interactions of semaglutide. Through a comprehensive in silico screening of semaglutide’s structural properties against a diverse panel of proteins, we have identified calmodulin (CaM) as a putative novel target of semaglutide. Molecular docking simulations revealed a strong interaction between semaglutide and CaM, characterized by favourable binding energies and a stable binding pose. Further molecular dynamics simulations confirmed the stability of the semaglutide–CaM complex, emphasizing the potential for a physiologically relevant interaction. In conclusion, our ligand-based drug design approach has uncovered calmodulin as a potential novel target of semaglutide. This discovery sheds light on the complex pharmacological profile of semaglutide and offers a promising direction for further research into the development of innovative therapeutic strategies for metabolic disorders. The CaM, and especially so the CaMKII, system is central in the experience of both drug- and natural-related reward. It is here hypothesized that, due to semaglutide binding, the reward pathway-based calmodulin system may be activated, and/or differently regulated. This may result in the positive semaglutide action on appetitive behaviour. Further studies are required to confirm these findings.
Full article
(This article belongs to the Topic Bioinformatics in Drug Design and Discovery, 2nd Volume)
►▼
Show Figures
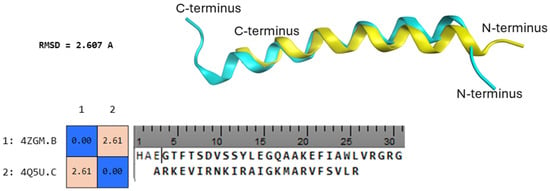
Figure 1
Open AccessReview
Complementary Practices in Pharmacy and Their Relation to Glaucoma—Classification, Definitions, and Limitations
by
Tibor Rák and Adrienne Csutak
Sci. Pharm. 2024, 92(1), 16; https://doi.org/10.3390/scipharm92010016 - 14 Mar 2024
Abstract
Background: Traditional and evidence-based medicines, as seen depicted throughout human history, reportedly first begin with the application of medicinal plants, animal products, or inorganic minerals as a basic framework towards effectively engineering the prototypes generally aligned to pharmaceuticals and medical nutrition. The growing
[...] Read more.
Background: Traditional and evidence-based medicines, as seen depicted throughout human history, reportedly first begin with the application of medicinal plants, animal products, or inorganic minerals as a basic framework towards effectively engineering the prototypes generally aligned to pharmaceuticals and medical nutrition. The growing global trend of complementary treatments for glaucoma can be explained by the intraocular pressure (IOP)-independent mechanisms of the disease and its interpretation as a progressive neurodegenerative disorder. Unfortunately, the categorical positions of the major fields of applied popular complementary therapies and their relation to glaucoma are consistently neglected. Methods: In consideration of bibliographic resources, the most well-known online scientific databases were searched. Conclusion: The rising popularity and the trends of products coming onto the market cannot escape the attention of pharmacists and ophthalmologists, as their patients suffering from eye diseases are also increasingly looking for such medicinal products. Most of them still lack knowledge of the appropriate evidence and side effect profiles. Our proposed systematic charts demonstrate the position of each mainstream complementary therapy throughout the applied medical sciences and are distinctively unique; we could not find any similar relevant illustration or resource among the published international literature.
Full article
(This article belongs to the Special Issue Feature Papers in Scientia Pharmaceutica)
►▼
Show Figures
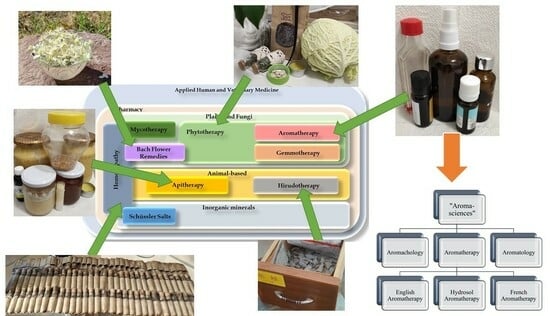
Graphical abstract
Open AccessArticle
Pharmacokinetic Simulation Study: Exploring the Impact of Clinical Parameters on Lamotrigine for Different Patient Populations with Implications for Liver Function Assessment and Therapeutic Drug Monitoring
by
Bárbara Costa, Isabel Silva, José Carlos Oliveira, Henrique Reguengo and Nuno Vale
Sci. Pharm. 2024, 92(1), 15; https://doi.org/10.3390/scipharm92010015 - 28 Feb 2024
Abstract
Lamotrigine, widely used for managing epilepsy and bipolar disorder, carries potential side effects, including severe anticonvulsant hypersensitivity syndrome (AHS) or drug rash with eosinophilia and systemic symptoms (DRESS), which may lead to hepatotoxicity. Patients with Type 2 Diabetes (TD2) and Non-Alcoholic Fatty Liver
[...] Read more.
Lamotrigine, widely used for managing epilepsy and bipolar disorder, carries potential side effects, including severe anticonvulsant hypersensitivity syndrome (AHS) or drug rash with eosinophilia and systemic symptoms (DRESS), which may lead to hepatotoxicity. Patients with Type 2 Diabetes (TD2) and Non-Alcoholic Fatty Liver Disease (NAFLD) are identified as more susceptible to these adverse reactions. This exploratory analysis aims to identify clinical parameters influencing lamotrigine pharmacokinetics across diverse populations, shedding light on toxicity and therapeutic drug monitoring (TDM) considerations. Starting with a retrospective analysis of 41 lamotrigine-treated patients at Hospital Santo António reveals changes or deviations from normal levels in various blood parameters and significant correlations between these parameters. Serum level changes, including creatinine, albumin, gamma-glutamyl transferase, total bilirubin, and Vitamin B12, are observed, with strong negative correlations between Vitamin B12 and creatinine. Then, we used GastroPlus and DILIsym to explore the impact of clinical parameters on lamotrigine for different patient populations. We constructed a Physiologically Based Pharmacokinetic (PBPK) model for lamotrigine in GastroPlus, based on ADMET predictions and data from the literature, to simulate the pharmacokinetic variability of lamotrigine in different populations, and we visualized the impact of increasing lamotrigine dose on its plasma concentration–time profiles (200 mg, 400 mg, 600 mg, 1200 mg) and reduced bioavailability. At higher doses, it is possible that the saturation of metabolic pathways leads to the formation of toxic metabolites or intermediates. These metabolites may exert inhibitory effects on drug-metabolizing enzymes or disrupt normal physiological processes, thereby impeding the drug’s clearance and potentially lowering its bioavailability. In DILIsym, we investigated lamotrigine’s DILI potential for individuals with diabetes and NAFLD. The results demonstrated an increased risk, emphasizing the need for careful monitoring. This study underscores the importance of understanding lamotrigine’s pharmacokinetics for tailored treatment decisions, improved outcomes, and minimized adverse reactions.
Full article
(This article belongs to the Special Issue Feature Papers in Scientia Pharmaceutica)
►▼
Show Figures
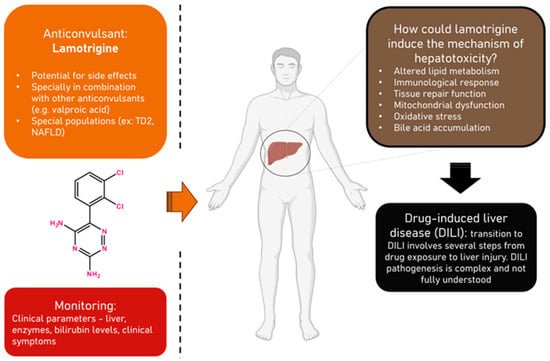
Figure 1
Open AccessArticle
New Carriers for Bioadhesive Gastroretentive Drug Delivery Systems Based on Eudragit® EPO/Eudragit® L100 Interpolyelectrolyte Complexes
by
Daria S. Gordeeva, Aleksandra V. Sitenkova (Bukhovets) and Rouslan I. Moustafine
Sci. Pharm. 2024, 92(1), 14; https://doi.org/10.3390/scipharm92010014 - 22 Feb 2024
Abstract
The aim of this study was the analysis of interpolyelectrolyte complexes (IPECs) based on Eudragit® EPO and Eudragit® L100 as prospective carriers for gastroretentive drug delivery systems (GRDDS) using two model drugs: metronidazole (MZ) and acyclovir (ACR). Eudragit® EPO/L100 IPECs
[...] Read more.
The aim of this study was the analysis of interpolyelectrolyte complexes (IPECs) based on Eudragit® EPO and Eudragit® L100 as prospective carriers for gastroretentive drug delivery systems (GRDDS) using two model drugs: metronidazole (MZ) and acyclovir (ACR). Eudragit® EPO/L100 IPECs with different pH concentrations were characterized by different degrees of swelling in mimicking fasted stomach medium (0.1 M HCl) and saved their shape for 6 h. The microenvironmental changes in IPEC structures in acidic medium were investigated using FT-IR spectroscopy, thermal and elemental analysis. IPEC samples showed bioadhesive properties that were not significantly different from the positive control (Carbopol) in the test with the mucin compacts. The release rate of metronidazole (class I BCS) from IPEC matrices increased with the increasing degree of swelling. IPEC 1 provided 49.62 ± 6.20% and IPEC 2 reached 87.69 ± 5.15% of metronidazole release after 6 h in mimicking fasted stomach medium (0.1 M HCl). The total amount of released acyclovir (class III BCS) from IPEC 1 was 25.76 ± 5.67% and from IPEC 2 was 21.48 ± 5.00%. Release of both drugs was controlled by relaxation of polymeric chains in matrices according to the Peppas–Sahlin model. According to the received results, investigated interpolymer complexes are prospects for further evaluation as carriers for gastroretentive bioadhesive systems.
Full article
(This article belongs to the Special Issue Feature Papers in Scientia Pharmaceutica)
►▼
Show Figures
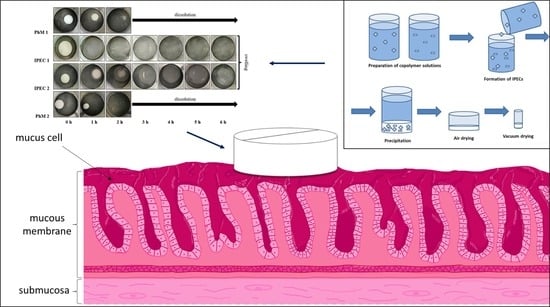
Graphical abstract
Open AccessArticle
Protective Effect of Panicum dichotomiflorum in a Rodent Model of Testosterone-Induced Benign Prostatic Hyperplasia
by
Eun Bok Baek, Eun-Ju Hong, Jung-Hee Kim, Min Kim, Jongmin Ahn and Hyo-Jung Kwun
Sci. Pharm. 2024, 92(1), 13; https://doi.org/10.3390/scipharm92010013 - 19 Feb 2024
Abstract
Benign prostatic hyperplasia (BPH) is a common disease in aging men. Panicum dichotomiflorum (PD) is an annual grass species of Poaceae that is distributed worldwide. The present study examined whether PD has a protective effect against BPH. BPH was generated in rats by
[...] Read more.
Benign prostatic hyperplasia (BPH) is a common disease in aging men. Panicum dichotomiflorum (PD) is an annual grass species of Poaceae that is distributed worldwide. The present study examined whether PD has a protective effect against BPH. BPH was generated in rats by daily subcutaneous administration of testosterone for four weeks. During this period, the rats were also given daily oral gavages of an extract of PD (150 mg/kg). After the final treatment, all animals were euthanized and their prostates were collected and weighed. In BPH model rats, the prostate weight and levels of dihydrotestosterone (DHT) and 5α-reductase expression were inhibited following treatment with PD extract. Testosterone-induced increases in prostate gland epithelial thickness and expression of cyclin D1 and proliferating cell nuclear antigen (PCNA) were markedly suppressed in PD-treated rats, whereas cleaved caspase-3 levels were increased. PD administration also decreased the expression of transforming growth factor (TGF)-β and vascular endothelial growth factor (VEGF), the phosphorylation of Akt, and inflammatory cytokines levels. Taken together, these results show that PD extract protects against testosterone-induced BPH progression by alleviating prostate cell growth and reducing levels of growth factors and inflammatory cytokines, indicating that PD extract may have potential in protecting against BPH.
Full article
(This article belongs to the Topic Natural Products and Drug Discovery)
►▼
Show Figures

Figure 1
Open AccessArticle
Attenuation of Pulmonary Damage Associated with COPD in a Cadmium-Exposed Model Due to the Administration of a siRNA Targeting PAD4
by
Sergio Adrian Ocampo-Ortega, Sandra Edith Cabrera-Becerra, Vivany Maydel Sierra-Sanchez, Vanessa Giselle García-Rubio, Citlali Margarita Blancas-Napoles, Rodrigo Romero-Nava, Fengyang Huang, Enrique Hong, Asdrúbal Aguilera-Méndez and Santiago Villafaña
Sci. Pharm. 2024, 92(1), 12; https://doi.org/10.3390/scipharm92010012 - 04 Feb 2024
Abstract
Chronic obstructive pulmonary disease (COPD), characterised by persistent airflow limitation during breathing, is considered to be the third leading cause of death worldwide. Among the mechanisms involved in this pathology is the excessive generation of neutrophil extracellular traps (NETs), which can induce an
[...] Read more.
Chronic obstructive pulmonary disease (COPD), characterised by persistent airflow limitation during breathing, is considered to be the third leading cause of death worldwide. Among the mechanisms involved in this pathology is the excessive generation of neutrophil extracellular traps (NETs), which can induce an unwanted inflammatory response. These traps have been reported to be generated by the enzyme peptidyl arginine deiminase 4 (PAD4). The aim of this work is therefore to evaluate the effect of the administration of a siRNA targeting PAD4 on lung damage in a COPD animal model. Wistar rats weighing 300–350 g were administered cadmium chloride (5 mg/kg i.p.) every 24 h. Then, following one week of the administration of cadmium chloride, the PAD4-targeted siRNA was administered, and at the second week, lung function was measured, as were lung and heart weights, as well as PAD4 expression by RT-PCR. Our results showed that cadmium administration generated a COPD model, which increased PAD4 expression and decreased lung and heart weights and respiratory function. SiRNA administration partially reversed the changes associated with the COPD model. In conclusion, our results suggest that administration of an siRNA targeting PAD4 could improve respiratory function by decreasing lung and heart damage.
Full article
(This article belongs to the Special Issue Feature Papers in Scientia Pharmaceutica)
►▼
Show Figures
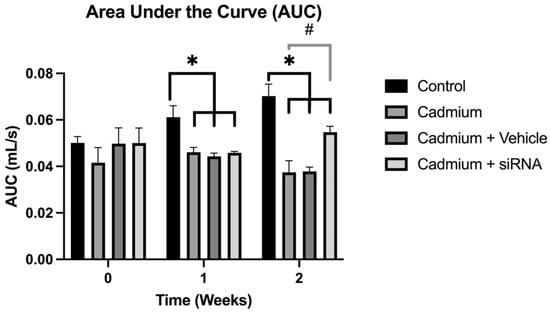
Figure 1
Open AccessArticle
The Potential of Incorporating a Pharmacist-Only Medicine Category in Poland
by
Tomasz Zaprutko, Józefina Sprawka, Barbara Maciuszek-Bartkowska, Piotr Ratajczak, Dorota Kopciuch, Anna Paczkowska and Krzysztof Kus
Sci. Pharm. 2024, 92(1), 11; https://doi.org/10.3390/scipharm92010011 - 04 Feb 2024
Abstract
Pharmacists play an important role, being increasingly focused on patient care and pharmaceutical services. This trend is also noticeable in Poland. Thus, we aimed to study the opinions of Polish pharmacists to determine the potential for introducing a new category of pharmacist-only medicines
[...] Read more.
Pharmacists play an important role, being increasingly focused on patient care and pharmaceutical services. This trend is also noticeable in Poland. Thus, we aimed to study the opinions of Polish pharmacists to determine the potential for introducing a new category of pharmacist-only medicines (POMs). This study was conducted during the COVID-19 pandemic. Hence, the survey (anonymous questionnaire consisting of 10 questions addressed to pharmacists) was only available in electronic form. A total of 500 correctly completed surveys were collected and subjected to further analysis. The vast majority of pharmacists (91.8%) revealed a willingness to expand their professional rights and 88% stated that the POMs implementation would be important. As a substance that should function as a POM instead of an OTC medicine, respondents most often indicated ketoprofen, sildenafil, and mometasone, accounting for 26.2%, 24.8%, and 24.4% of responses, respectively. In terms of funding pharmaceutical services, 54.2% of respondents indicated that costs should be covered partially by the patient and the payer. There is a clear need for the incorporation of the POM category in Poland. Polish pharmacists are anticipating the development of pharmaceutical services which should be partly covered by patients and payers.
Full article
(This article belongs to the Special Issue Feature Papers in Scientia Pharmaceutica)
►▼
Show Figures
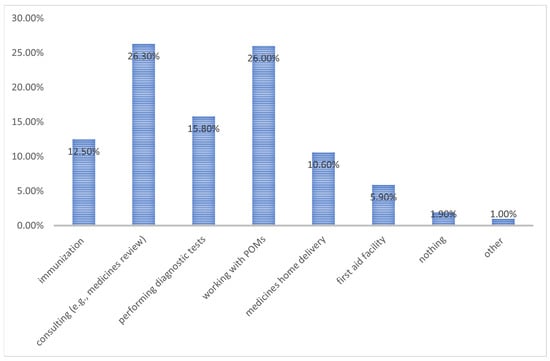
Figure 1
Open AccessArticle
Mucoadhesive Vaginal Tablets Containing Metronidazole: Screening of Optimal Natural Polymer in the Composition
by
Kamila Bartoníková, Miroslava Špaglová, Martina Papadakos, Michal Hanko and Oliver Macho
Sci. Pharm. 2024, 92(1), 10; https://doi.org/10.3390/scipharm92010010 - 26 Jan 2024
Abstract
►▼
Show Figures
(1) Background: The study aimed to compare the impact of various natural polymers–sodium alginate, acacia gum, carrageenan, guar gum, xanthan gum, and tragacanth on the formulation and the physical properties of mucoadhesive vaginal tablets containing metronidazole (167 mg/g). (2) Methods: The quality of
[...] Read more.
(1) Background: The study aimed to compare the impact of various natural polymers–sodium alginate, acacia gum, carrageenan, guar gum, xanthan gum, and tragacanth on the formulation and the physical properties of mucoadhesive vaginal tablets containing metronidazole (167 mg/g). (2) Methods: The quality of the tablets prepared by direct compression was evaluated by pharmacopoeia tests (uniformity of mass, resistance to crushing, friability). Mucoadhesion of the tablets was characterized by swelling capacity and mucoadhesive strength, i.e., the force required to detach the tablet from the rabbit mucosa. In vitro drug release was performed by a modified dissolution method in paddle apparatus containing the simulated vaginal fluid (pH 4.5). Scanning electron microscopy observed morphological changes on the swollen tablets’ surface. (3) Results: Pharmacopoeia tests have shown that all prepared tablets met the requirements on quality. The highest mucoadhesive strength was noted in tablets containing guar and xanthan gum. The highest swelling capacity was possessed by tablets containing carrageenan. (4) Conclusions: Summarizing all tests’ results, sodium alginate can be considered the most suitable natural polymer in tablet formulation. The combination of polymers providing higher mucoadhesiveness and at the same time a prolonged release, e.g., xanthan or guar, together with sodium alginate, could also be of interest.
Full article
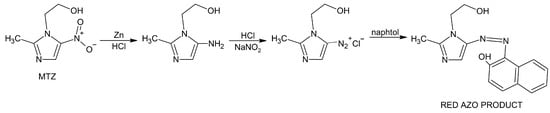
Figure 1
Open AccessArticle
Effect of Zoapatle (Montanoa tomentosa) on Inflammatory Markers in a Murine Model of Ventricular Hypertrophy
by
Carlos Enrique López-Luna, Cruz Vargas-De-León, Rocio Alejandra Gutiérrez-Rojas, Karla Aidee Aguayo-Cerón, Claudia Camelia Calzada-Mendoza, Fengyang Huang, Rodrigo Romero-Nava and Maria Esther Ocharan-Hernandez
Sci. Pharm. 2024, 92(1), 9; https://doi.org/10.3390/scipharm92010009 - 24 Jan 2024
Abstract
Zoapatle, a native plant utilized for centuries in traditional Mexican medicine, is abundantly found in Mesoamerica and northern South America. Pleiotropic effects of this genus have been recognized, primarily inducing alterations in smooth muscle contractility in animal models. The aim of this study
[...] Read more.
Zoapatle, a native plant utilized for centuries in traditional Mexican medicine, is abundantly found in Mesoamerica and northern South America. Pleiotropic effects of this genus have been recognized, primarily inducing alterations in smooth muscle contractility in animal models. The aim of this study was to evaluate the effect of Zoapatle on the hypertrophy index and the gene expression of TNF-α, IL-1β, NF-κB, STAT5, and the PRLR in the brain, left ventricle, and renal cortex of rats with isoproterenol-induced cardiac hypertrophy. Three groups were studied, the control group (n = 4), hypertrophy group (n = 4) and hypertrophy group treated with Zoapatle (n = 4). A ventricular hypertrophy model was developed with 150 mg/kg/day of isoproterenol intraperitoneally administered over two days with a 24 h interval between applications. Zoapatle was administered for 28 consecutive days (25 mg/kg). Gene expression was determined with RT-qPCR. Subsequently, a principal component analysis (PCA) was performed using the RNA expression variables. A notably reduced left ventricle mass index was observed in the Zoapatle group. Additionally, Zoapatle administration in cardiac hypertrophy demonstrated a significant decrease in the gene expression of TNF-α, IL-1B, STAT 5, and the PRLR. TNF-α and the transcription factor STAT5 exhibited a similar trend in both the left ventricle and renal cortex, suggesting a correlation with the inflammatory state in these tissues due to ventricular hypertrophy. The findings suggest that Zoapatle reverses the hypertrophy index in a hypertrophy model, concurrently reducing several proinflammatory mediators associated with the hypertrophy index.
Full article
(This article belongs to the Topic Natural Products and Drug Discovery)
►▼
Show Figures
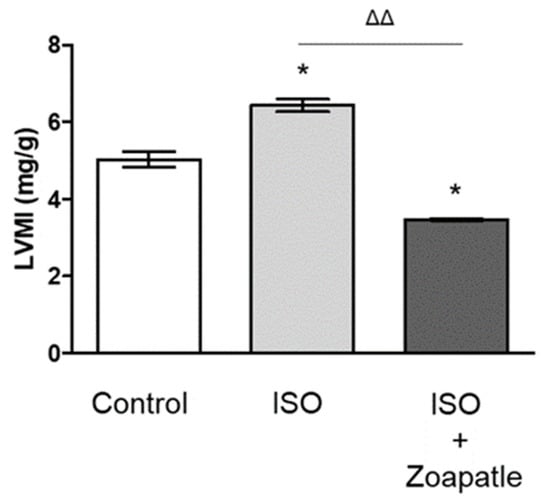
Figure 1
Open AccessArticle
A Thermal-Analysis-Technique-Based Mechanistic Approach toward the Release of Omeprazole from Solid Dosage Forms
by
Georgios Agapakis, Angeliki Siamidi, Stefanos Kikionis, Marilena Vlachou and Natassa Pippa
Sci. Pharm. 2024, 92(1), 8; https://doi.org/10.3390/scipharm92010008 - 16 Jan 2024
Abstract
►▼
Show Figures
The design, development, and release kinetics of omeprazole (OME) from solid dosage forms have been investigated. These formulations were examined for their resilience in pH = 4.5 buffer solutions and their rate of disintegration in a small-intestine-like environment (pH = 6.8). The results
[...] Read more.
The design, development, and release kinetics of omeprazole (OME) from solid dosage forms have been investigated. These formulations were examined for their resilience in pH = 4.5 buffer solutions and their rate of disintegration in a small-intestine-like environment (pH = 6.8). The results were compared with those of the well-known brand product Losec®, where its use is accompanied by numerous benefits but drawbacks as well. Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) tests were conducted in order to examine the release kinetics of the various dosage forms and provide explanations based on the interactions between the excipients and the active substance.
Full article
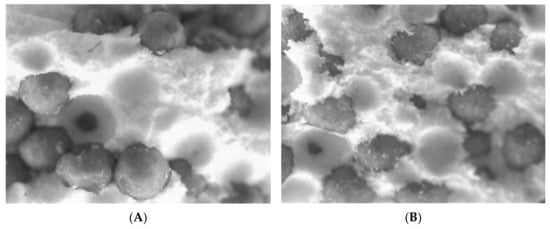
Figure 1
Open AccessReview
The Risks of “Getting High” on Over-the-Counter Drugs during Pregnancy
by
Bianca-Eugenia Ősz, Ruxandra Ștefănescu, Andreea Sălcudean, George Jîtcă and Camil-Eugen Vari
Sci. Pharm. 2024, 92(1), 7; https://doi.org/10.3390/scipharm92010007 - 09 Jan 2024
Abstract
►▼
Show Figures
Easy access to over-the-counter (OTC) drugs makes it possible to procure active substances that normally used in therapeutic doses do not raise health problems. The use of high doses of OTC drugs containing codeine, loperamide, pseudoephedrine, diphenhydramine or dimenhydrinate, as well as the
[...] Read more.
Easy access to over-the-counter (OTC) drugs makes it possible to procure active substances that normally used in therapeutic doses do not raise health problems. The use of high doses of OTC drugs containing codeine, loperamide, pseudoephedrine, diphenhydramine or dimenhydrinate, as well as the use of benzidamine systemically raises concerns regarding the increase in units sold. These drugs are used for recreational or euphorizing purposes, including by young women of childbearing age, psychoactive substance users representing a risk group in terms of the possibility of an unplanned pregnancy. Abusive consumption of OTC products during pregnancy is harmful, with consequences for both fetal and late development that can occur in the infant. This literature review presents the risks (teratogenicity, fetal toxicity, neonatal abstinence syndrome, etc.) associated with the use of potentially psychoactive OTC drugs to emphasize the importance of re-evaluating OTC classification and dispensing.
Full article
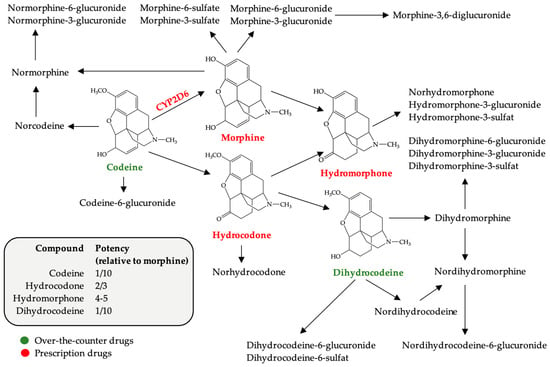
Figure 1
Open AccessReview
The Extraction of Bioactive Agents from Calophyllum inophyllum L., and Their Pharmacological Properties
by
Sahena Ferdosh
Sci. Pharm. 2024, 92(1), 6; https://doi.org/10.3390/scipharm92010006 - 09 Jan 2024
Abstract
Calophyllum inophyllum L. has been used for many generations by Pacific Islanders because of its numerous health and aesthetic advantages. The leaves, stems, roots, fruits, flowers, and seeds of this plant contain significant phytochemicals, including flavonoids, coumarins, fatty acids, and xanthones, which have
[...] Read more.
Calophyllum inophyllum L. has been used for many generations by Pacific Islanders because of its numerous health and aesthetic advantages. The leaves, stems, roots, fruits, flowers, and seeds of this plant contain significant phytochemicals, including flavonoids, coumarins, fatty acids, and xanthones, which have been shown to have wound healing, analgesic, anti-inflammatory, antiaging, anti-arthritic, anti-cancer, anti-proliferative, anti-diabetic, anti-microbial, and anti-HIV effects. The chemical profiles and bioactive potential may vary due to different extraction techniques, plant parts, and geographical origins. Extraction is the essential first step in the analysis of bioactive compounds that leads to further separation, identification, and characterization. Conventional methods like maceration, Soxhlet, and percolation are often used to extract bioactive compounds from C. inophyllum. However, little study has been carried out on non-conventional methods such as pressured liquid extraction, supercritical fluid extraction (SFE), and ultrasound-assisted extraction. The SFE method can be used to extract bioactive compounds from C. inophyllum to retain their pharmacological properties for application in pharmaceutical and cosmetic products.
Full article
Open AccessArticle
Diastereomers of Spheroidal Form and Commercially Available Taxifolin Samples
by
Roman P. Terekhov, Evgeny S. Melnikov, Ilya D. Nikitin, Margarita A. Tokareva, Tatyana A. Rodina, Anastasiya D. Savina, Denis I. Pankov, Anastasiya K. Zhevlakova, Vladimir L. Beloborodov and Irina A. Selivanova
Sci. Pharm. 2024, 92(1), 5; https://doi.org/10.3390/scipharm92010005 - 03 Jan 2024
Abstract
Taxifolin is a natural polyphenol belonging to the class of flavonoids. The structure of this compound is characterized by the presence of two chiral centers. The spheroidal form of taxifolin (TAXs) has emerged as a promising modification due to enhanced solubility, higher safety
[...] Read more.
Taxifolin is a natural polyphenol belonging to the class of flavonoids. The structure of this compound is characterized by the presence of two chiral centers. The spheroidal form of taxifolin (TAXs) has emerged as a promising modification due to enhanced solubility, higher safety profile, and long-term release from solid dosage forms. The study’s objective was to assess the diastereomeric content in TAXs and industrially produced samples of taxifolin. Considering the difference in the physico-chemical properties of diastereomers and based on the literature data, we developed a qualitative HPLC method. The chromatograms were recorded using a diode array detector at 290 nm and a mass spectrometer operated in negative ionization mode. Our data suggest that a biphenyl column and gradient elution using 0.1% formic acid in water and 0.2% formic acid in methanol, with the organic phase gradient from 7% to 21% and a flow rate of 0.65 mL/min for 15 min at 60 °C, provides the best conditions for the separation of taxifolin diastereomers. This method was validated for quantitative analysis. We discovered that the cis-isomer was present in all the analyzed samples, with its quantity ranging from 0.8% to 9.5%. TAXs can be considered a sample enriched with diastereomers.
Full article
(This article belongs to the Topic Natural Products and Drug Discovery)
►▼
Show Figures
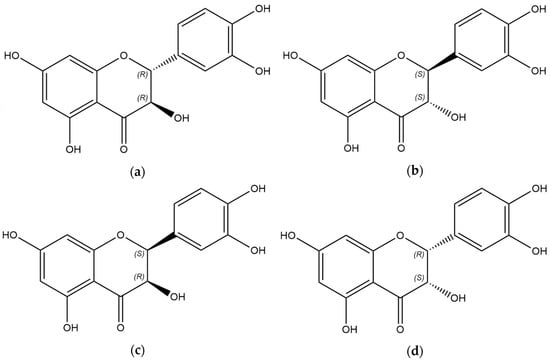
Figure 1
Open AccessArticle
Assessing the Influence of a Rotating Magnetic Field on Ibuprofen Permeability from Diverse Pharmaceutical Formulations
by
Anna Nowak, Paula Ossowicz-Rupniewska, Maciej Konopacki, Anna Muzykiewicz-Szymańska, Łukasz Kucharski and Rafał Rakoczy
Sci. Pharm. 2024, 92(1), 4; https://doi.org/10.3390/scipharm92010004 - 29 Dec 2023
Abstract
►▼
Show Figures
This study introduces a novel approach for enhancing the transdermal permeability of ibuprofen through the skin by utilising a rotating magnetic field (RMF). The core objective is to systematically evaluate the influence of a 50 Hz RMF on ibuprofen’s skin permeability across various
[...] Read more.
This study introduces a novel approach for enhancing the transdermal permeability of ibuprofen through the skin by utilising a rotating magnetic field (RMF). The core objective is to systematically evaluate the influence of a 50 Hz RMF on ibuprofen’s skin permeability across various formulation types, each employing distinct physical forms and excipients. The experimental setup involved Franz cells with skin as the membrane, exposed to a 50 Hz RMF in conjunction with specific formulations. Subsequent comprehensive analysis revealed a notable increase in the transdermal transport of ibuprofen, irrespective of the formulation employed. Notably, the differences in the initial 30 min of permeation were particularly pronounced. Crucially, this investigation establishes that the application of a 50 Hz RMF resulted in a remarkable over-sevenfold increase in ibuprofen permeability compared to the control group without RMF exposure. It is noteworthy that in all semi-solid pharmaceutical formulations tested, RMF effectively reduced the delay time to zero, underscoring the efficiency of RMF in overcoming barriers to transdermal drug delivery. This research positions the application of RMF as a highly promising and innovative technology, significantly enhancing the transdermal penetration of anti-inflammatory and analgesic drugs through the skin. The demonstrated effectiveness of RMF across diverse formulations suggests its potential in transdermal drug delivery, offering a novel and efficient strategy for improving therapeutic outcomes in the administration of ibuprofen and potentially other pharmaceutical agents.
Full article
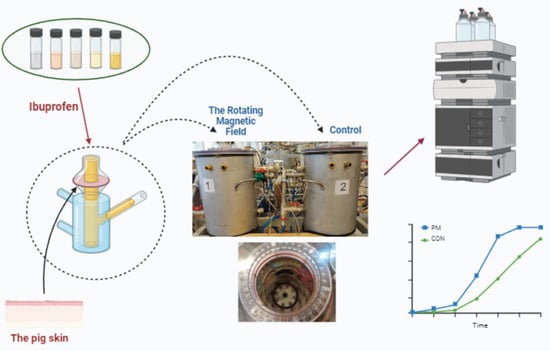
Graphical abstract

Journal Menu
► ▼ Journal MenuJournal Browser
► ▼ Journal BrowserHighly Accessed Articles
Latest Books
E-Mail Alert
News
Topics
Topic in
Biomedicines, JCM, Molecules, Pharmaceutics, Sci. Pharm., IJMS
Cannabis, Cannabinoids and Its Derivatives
Topic Editors: Melanie Kelly, Christian LehmannDeadline: 30 September 2024
Topic in
Biomedicines, Biomolecules, IJMS, Sci. Pharm., Plants, Foods, Molecules
Natural Products and Drug Discovery
Topic Editors: Marta Menegazzi, Sonia PiacenteDeadline: 31 December 2024
Topic in
BioChem, Biomolecules, CIMB, Molecules, Pharmaceutics, Sci. Pharm.
Design, Synthesis and Biological Evaluation of Novel Small Molecules as Multi-target Enzyme Inhibitors
Topic Editors: Davide Moi, Daniele Passarella, Andrea CitarellaDeadline: 31 January 2025
Topic in
Antibiotics, IJMS, Molecules, Pharmaceutics, Sci. Pharm.
Designing New Antimicrobials Based on Known Valuable Heterocycles as Building Blocks
Topic Editors: Aura Rusu, Gabriel Hancu, Vladimír GarajDeadline: 31 March 2025





